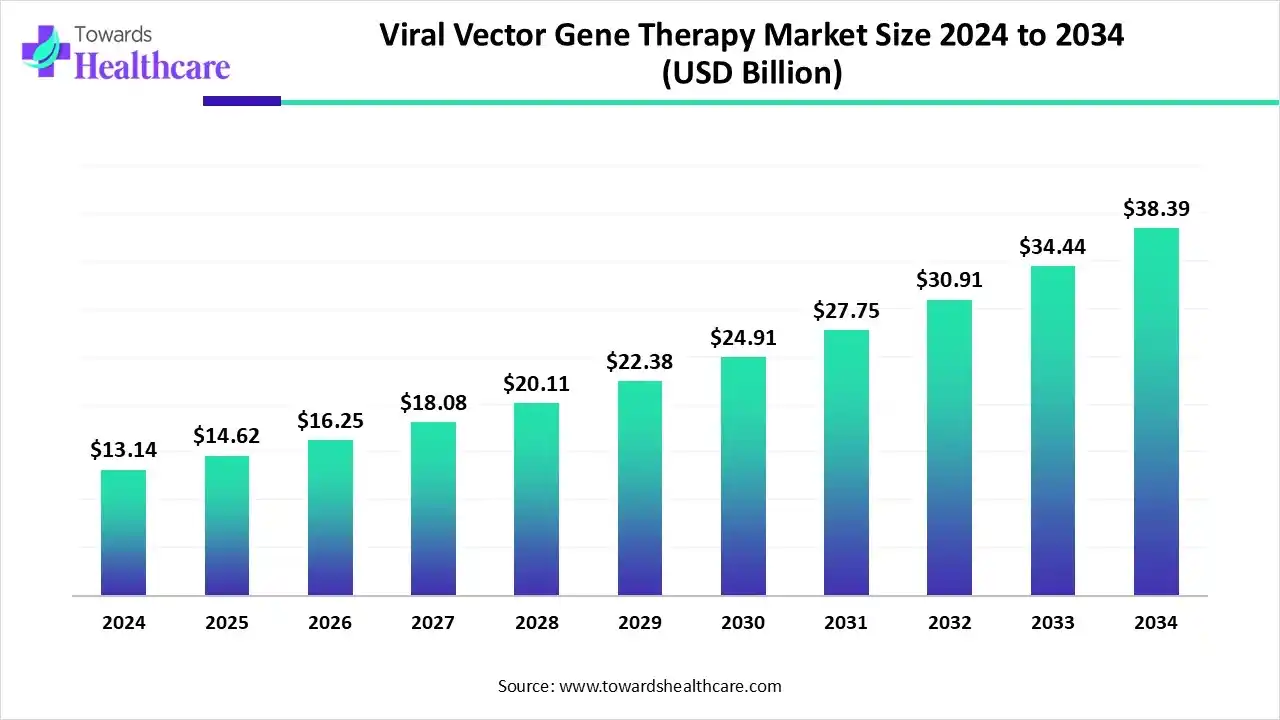
Viral Vector Gene Therapy Market Size Expects to Reach USD 38.39 Billion by 2034
The viral vector gene therapy market size is calculated at USD 14.62 billion in 2025 and is expected to reach around USD 38.39 billion by 2034, growing at a CAGR of 11.23% for the forecasted period.
Ottawa, Oct. 06, 2025 (GLOBE NEWSWIRE) -- The global viral vector gene therapy market size was valued at USD 13.14 billion in 2024 and is predicted to hit around USD 38.39 billion by 2034, rising at a 11.23% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
This market is rising due to breakthroughs in vector design, rising prevalence of genetic and rare diseases, and strong R&D investment are creating a fertile environment for expansion in viral vector gene therapy.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5737
Key takeaways:
- North America dominated the viral vector gene therapy market in 2024.
- Asia-Pacific is expected to grow at the highest CAGR in the market during the forecast period.
- By vector type, the adeno-associated virus segment dominated the market.
- By vector type, the lentivirus segment is expected to grow at the fastest CAGR in the market during the studied years.
- By application, the gene therapy segment held the largest revenue market share and is expected to grow at the fastest CAGR in the market during the studied years.
- By end users, the pharmaceutical and biotechnology companies segment led the market.
- By end user, the Contract Research Organizations (CROs) & Contract Manufacturing Organizations (CMOs) segment is expected to grow at the fastest CAGR in the market during the studied years.
Market Overview
What is driving the growth of the viral vector gene therapy market?
The global viral vector gene therapy market is growing rapidly due to companies developing and researching gene therapy treatments for genetic disorders. The growth of the market can be attributed to increasing clinical approvals of new therapies, more regulatory decisions allowing advanced therapies to proceed and expanded interest in curative medicine and precision medicine. Ongoing development of vector modification (improved safety, targeting, and payload capacity) and new large-scale manufacturing platforms have also advanced the field.
Moreover, the growing awareness and diagnosis of rare diseases, together with increased public and private funding, provide additional acceleration to the pipeline. By allowing the development of more effective (and durable) therapeutics for previously untreatable maladies, viral vector gene therapies are poised to be a foundational aspect of the next generation of medical care.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What is driving growth in the viral vector gene therapy market?
- Advancements in viral vector engineering and delivery technologies: Continuous optimization of viral capsids, promoter systems, and tissue-targeting approaches increases transduction efficiency and minimizes immunogenicity, providing for safer and more effective therapies, respectively.
- Increasing incidence and awareness of genetic, rare, and chronic diseases: Expanded diagnostic capabilities (sequencing, newborn screenings) can now detect more patients with monogenic conditions, which supports the use of gene therapies that address the underlying cause.
- Strong financing and regulatory support: Government, venture capital, and pharma industries are investing considerable resources to fund gene therapy R&D, regulatory agencies are providing assistance and pathways (e.g. orphan drug designation, expedited review) to facilitate clinical studies.
- Rising momentum for outsourcing and modular manufacturing capabilities: The complexity of viral vector production is a core reason for pharmaceutical companies to utilize contract research organizations (CROs) and pharma contract manufacturing organizations (CMOs), driving the need for external manufacturing capacity and capabilities.
Which trends are changing the viral vector gene therapy industry?
- One emerging trend is the growing adoption of AI and machine learning to improve vector design, capsid engineering, and predictive safety modeling which increase development success.
- Another trend is the development of integrated end-to-end manufacturing platforms in a single facility or vertically integrated partners which reduces transfer inefficiencies and shortens the period to scale-up.
- In addition, there are continued developments in state-of-the-art delivery methods to translate into therapeutic reach into difficult-to-deliver organs such as the CNS or ocular tissues.
Major Challenge:
Manufacturing Complexity and Scalability Constraints:
As one of the biggest challenges to the viral vector gene therapy market, vector manufacturing is complex and expensive. Producing viral vectors at GMP grade requires trained personnel, strict quality control systems, expensive capital equipment, and extensive regulatory oversight. Furthermore, achieving scalability while maintaining acceptable levels of vector purity, potency, and reproducibility is complex and burdensome. In addition, supply chain limitations, and process-related losses, can drive up costs and impede overall capacity for production which slows the availability of therapies.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
Regional Insights:
North America is currently the leading region in the viral vector gene therapy market, with the largest market share, due to its robust biotech ecosystem, advanced research capacity, and early-stage adoption of innovative therapies. The U.S. continues to dominate in this region due to the high density of pharmaceutical companies, biotech start-ups, and academic research institutions providing research capacity in the development of viral vectors. Additionally, FDA designations, such as priority review and orphan drug market designation, further accelerate commercialization. The combination of ample venture capital and government funding, along with a growing patient population of rare and genetic disorders, reinforces North America's lead in the viral vector gene therapy market.
United States – Growth Drivers in the Viral Vector Gene Therapy Market
- Strong R&D Infrastructure: The U.S. leads in gene therapy research, supported by top biotech companies, academic institutions, and federal agencies.
- High Investment Volume: Significant venture capital and private equity investments are flowing into gene therapy start-ups and CDMOs.
- Advanced Regulatory Pathways: The FDA has created expedited pathways (e.g., Fast Track, RMAT) for cell and gene therapies, enabling quicker approvals.
- Presence of Leading CDMO Players: The U.S. hosts key players in viral vector manufacturing with advanced capabilities in lentiviral and AAV production.
- High Number of Clinical Trials: A large share of global gene therapy clinical trials are conducted in the U.S., reflecting strong market activity.
- Supportive Reimbursement Landscape: While evolving, the U.S. reimbursement ecosystem is becoming increasingly structured to accommodate high-cost gene therapies.
-
Growing Rare Disease and Oncology Pipeline: Demand is driven by therapies targeting rare genetic conditions and cancers.
Download the single region market report @ https://www.towardshealthcare.com/price/5737
Asia-Pacific is fast becoming the fastest growing region in the viral vector gene therapy market, thanks to swift improvement in healthcare infrastructure and investment in biotech. Be sure to highlight countries like China, India, Japan, and Korea and their investment in research collaborations, clinical trials, and domestic viral vector manufacture. Favorable government policies, increasing healthcare expenditures, and the growing prevalence of rare and genetic disorder all contribute to the increase in demand as well. Furthermore, Asia-Pacific offers cheaper manufacturing and clinical trial environments, creating an attractive option for global pharmaceutical companies.
India – Growth Trends in the Viral Vector Gene Therapy Market
- Emerging R&D Ecosystem: India is building its gene therapy research base, with increasing academic and public-private research partnerships.
- Lower Manufacturing Costs: Cost-efficient labor and production make India attractive for viral vector manufacturing and global outsourcing.
- Rising Government Support: Initiatives like National Biopharma Mission and policies supporting biotechnology are helping boost the sector.
- Growing Biotech Start-up Scene: Indian biotech companies are beginning to enter the gene therapy and viral vector space, some with global collaborations.
- Focus on Local CDMO Capacity Building: India is investing in setting up viral vector and biologics manufacturing infrastructure.
- Patient Pool & Unmet Need: A large population with untreated or rare genetic conditions creates significant long-term demand potential.
Segment Insights:
By vector type:
The adeno-associated virus (AAV) segment leads the viral vector gene therapy market, due to it being an exceptionally safe virus with widespread tissue tropism and a capacity to deliver genetic information devoid of integrating into the cellular genome. The combination of these attributes, particularly with AAV vectors, has made AAV the viral vector of choice in applications for both therapeutic strategies. AAV is especially advantageous for long-term administration in patients with genetic disease. Owing to its inherent low immunogenicity profile and capacity to traverse the blood-brain barrier, biotechnology that employs AAV has produced exciting therapeutic advancements in neurological and ocular diseases.
The lentivirus vector segment is growing at the fastest rate in the viral vector gene therapy market, primarily because lentivirus possesses the unique capacity to integrate into dividing and non-dividing cells, which allows for long-term stable expression of therapeutic genes. As such, lentivirus has great value in ex vivo settings such as cell-based therapies. In fact, lentiviruses, in contrast with non-integrating vectors, produce durable outcomes that are vital for successfully treating chronic and inherited diseases.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
By application:
The gene therapy application segment is the leading segment in the viral vector gene therapy market due to the essential role of viral vectors in delivering therapeutic gene to patient cells. Viral vectors serve as a vehicle to rectify or replace defective genes, hence having curative potential to a number of inherited and acquired diseases. The increase in instances of rare genetic disorders like haemophilia, spinal muscular atrophy, and select metabolic disorders has boosted demand for targeted gene therapies.
Viral vectors, especially adeno-associated viruses (AAVs) and lentiviruses, have demonstrated high efficacy and safety among clinical trials which has led to several regulatory approvals in recent years. Additionally, increased investment from pharma companies and extended research and development pipelines are increasing the velocity of new therapies in multiple indication areas like oncology, neurology and cardiology.
By End-User:
The viral vector gene therapy market's largest end-user segment is represented by pharmaceutical and biotechnology companies. This is mainly driven by innovation, product development, and commercialization. These companies make significant investments to take gene therapy products through research pipelines, clinical trials, and regulatory approvals. There are more patients with rare and genetic diseases today, resulting in leading pharma and biotech companies allocating substantial resources to develop viral vector treatments in different therapeutic applications, such as oncology, neurology, and metabolic diseases.
Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs) are the fastest growing end-user segment in the viral vector gene therapy market. Viral vector design can be complex, and it is increasingly challenging to comply with regulatory requirements and maintain in-house capabilities, which are costly endeavours. As a result, many pharma and biotech companies decide to outsource some aspects of work. CROs provide specific capabilities and expertise in preclinical and clinical trial design, regulatory submissions, and analytics. CMOs provide more advanced platforms and scalable capabilities to manufacture viral vectors under GMP conditions.
Recent Development:
In September 2025, DINAMIQS (part of Siegfried) officially opened a new cGMP viral vector manufacturing facility capable of end-to-end vector development and fill/finish, strengthening its presence in the gene therapy production value chain.
Browse More Insights of Towards Healthcare:
The U.S. viral vector and plasmid DNA manufacturing market was valued at US$ 2.85 billion in 2024, increasing to US$ 3.3 billion in 2025, and is projected to reach approximately US$ 12.31 billion by 2034, growing at a CAGR of 15.72% from 2025 to 2034.
The global viral vector-based cell & gene therapy CDMO market stood at US$ 142.77 million in 2024, rose to US$ 162 million in 2025, and is anticipated to reach around US$ 497.7 million by 2034, expanding at a CAGR of 13.44% during 2025–2034.
The global viral vector development market was valued at US$ 0.89 billion in 2024, increased to US$ 1.06 billion in 2025, and is expected to reach about US$ 5 billion by 2034, registering a CAGR of 18.84% between 2025 and 2034.
The global non-viral transfection reagents market was valued at US$ 1.85 billion in 2024, grew to US$ 2.01 billion in 2025, and is projected to hit nearly US$ 4.29 billion by 2034, advancing at a CAGR of 8.73% over the forecast period.
The global viral sensitizers market reached US$ 2.04 billion in 2024, increased to US$ 2.34 billion in 2025, and is forecasted to reach around US$ 8.02 billion by 2034, expanding at a CAGR of 14.63% between 2025 and 2034.
The global viral vaccine cell culture media market was valued at US$ 1.83 billion in 2024, grew to US$ 1.94 billion in 2025, and is anticipated to reach US$ 3.24 billion by 2034, reflecting a CAGR of 5.84% over the same period.
The global lentiviral vector market was valued at US$ 360 million in 2024, rose to US$ 426.82 million in 2025, and is projected to reach nearly US$ 1,943.24 million by 2034, growing at a CAGR of 18.56% from 2025 to 2034.
The global viral vector manufacturing market was valued at US$ 1.5 billion in 2024, grew to US$ 1.82 billion in 2025, and is expected to reach approximately US$ 10.53 billion by 2034, expanding at a robust CAGR of 21.64% over the forecast timeline.
The APAC viral vector and plasmid DNA manufacturing market was valued at US$ 1.38 billion in 2024, increased to US$ 1.68 billion in 2025, and is anticipated to reach around US$ 10.01 billion by 2034, advancing at a CAGR of 21.93% between 2025 and 2034.
Meanwhile, the global anti-viral nasal spray market is witnessing steady growth, with projections indicating significant revenue expansion, potentially reaching hundreds of millions in value between 2024 and 2034.
Overall, the global viral vector and plasmid DNA manufacturing market stood at US$ 6.01 billion in 2023 and is forecasted to reach approximately US$ 43.04 billion by 2034, registering a strong CAGR of 20.7% from 2024 to 2034.
Top Companies in the Viral Vector Gene Therapy Market
- Novartis AG
- Spark Therapeutics
- Merck KGaA
- Regenxbio
- Uniqure NV
- Cobra Biologics AB
- CRISPR Therapeutics
- Bluebird Bio
- Lonza Group AG
Download the Competitive Landscape market report @ https://www.towardshealthcare.com/price/5737
Segments Covered in the Report
By Vector Type
- Adeno-associated virus (AAV)
- Lentivirus
- Adenovirus
- Retrovirus
- Others
By Application
- Gene Therapy
- Vaccine Development
- Biopharmaceutical & Pharmaceutical Discovery
By End-User
- Pharmaceutical and Biotechnology Companies
- Academic and Research Institutes
- Contract Research Organizations (CROs) & Contract Manufacturing Organizations (CMOs)
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/price/5737
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
